The capability for invasion and metastasis acquired during tumor progression stands as the basis for much of the morbidity and mortality associated with cancer. It is also a remarkably diverse and complex parameter. We are studying various facets of this cancer phenotype, in multiple model systems, of which a few are highlighted below.
Invasiveness stimulated by forces in the tumor microenvironment
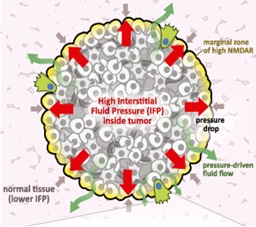
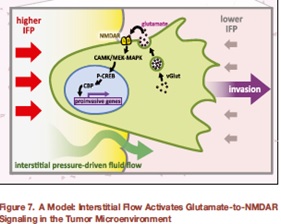
A PhD student discovered a regulatory pathway that activates invasiveness, schematized above, which is triggered by mechanical forces resultant to differences in hydrostatic pressure between solid tumors and their surrounding tissue, resulting in fluid flow out of the tumor. The pressure-driven fluid flow induces secretion of glutamate, which, in cancer cells expressing the neuronal signaling receptor NMDAR, activates it, stimulating downstream pathways – still to be defined – that elicit an invasive growth program (Li and Hanahan, 2013). We continue to investigate this invasive growth program, in both forms of pancreatic cancer, and in triple negative breast cancer, where it may define a heretofore unappreciated subtype that has coopted glutamate signaling to help drive the malignant phenotype.
microRNA dynamics governing the metastatic cascade
A previous study from the Hanahan lab implicated a set of microRNAs (miRs) in a comparatively rare subtype of pancreatic neuroendocrine cancer that is associated with metastasis (Olson et al 2010); the existence of this metastasis-prone subtype has recently been substantiated in the mouse model and in the cognate human cancer (Sadanandam, et al 2015). Interestingly, the evidence suggests that this metastasis-prone subtype has a distinct cell-of-origin, namely a pancreatic progenitor cell, as illustrated below.
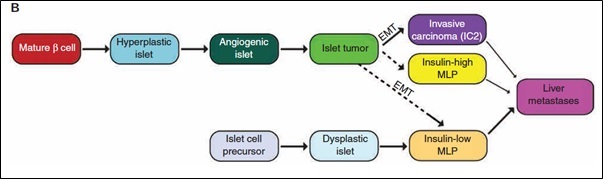
One current research project is unraveling the signature set of ‘pro-metastatic’ miRs. We have identified individual miRs that are contributing in complementary ways to the early invasive and intermediate seeding stages of metastasis, and are seeking to identify the genes (and pathways) that they regulate. An additional agenda is to identify miRs that govern the later stage involving colonization of macro-metastases.